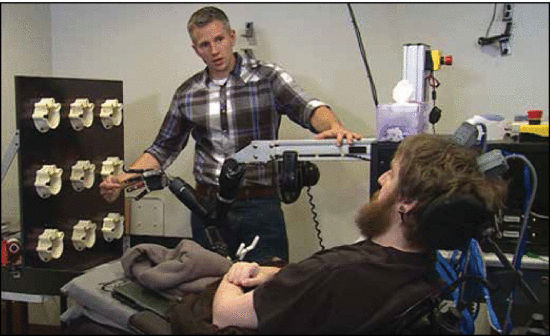
 Figure 1.
Microelectrode arrays placed in Nathan Copeland’s brain. Source: DARPA [19].
Figure 1.
Microelectrode arrays placed in Nathan Copeland’s brain. Source: DARPA [19]. Carolena Stephanie Larson and Muhammad Salar Khan
In the United States, approximately 2 million people live with limb loss [1] and that number is expected to double by 2050 [2]. Although this is a significant figure, it still does not represent a substantial market size for the private sector to develop prosthetics for this population. Mobility is highly crucial for long-term health [3], [4], [5], [6], and prosthetic limbs are vital in restoring mobility. Undoubtedly, people with limb loss are among the most vulnerable segments of society. Therefore, we contend that their safety and care should be a high priority for the government. In this article, we recap the public policy landscape surrounding prosthetic limbs in the United States before proposing ways to increase access and adoption of prosthetic limbs.
In the United States, two federal departments are involved in the creation, maturation, and diffusion of prosthetic limbs. The first is the Food and Drug Administration (FDA). The second, understandably, is the Department of Veterans Affairs (VA), as soldiers are at a high risk of losing limbs in combat duty. For prosthetic researchers, funding from traditional sources such as the National Institutes of Health (NIH) and the National Science Foundation (NSF) has become remarkably difficult to secure, partly because of the competition from other projects in those institutions [7]. Consequently, many researchers have turned their attention to the VA. In 2014–2015, the Congressionally Directed Medical Research Program (CDMRP) at the Department of Defense (DOD) funded 18 proposals in orthotics and prosthetics outcomes. However, obtaining such funding is also becoming more competitive, as 98 proposals were rejected [7].
For prosthetic researchers, funding has become difficult to secure.
In 1938, Congress gave the FDA the authority to oversee the safety of food, drugs, medical devices, and cosmetics through the Federal Food, Drug, and Cosmetic Act (FFDCA). Consequently, the FDA uses guidance documents to approve medical devices and radiation-emitting products (including prosthetics) for commercialization and distribution. While the FDA stimulates the diffusion of medical devices and prosthetics by ensuring they are safe for human use, the VA has been a driving force behind the invention and adoption of prosthetic limbs in service members (and veterans) and civilian populations alike.
The history of limb development dates back to the second half of the 19th century. Starting in 1862, Congress appropriated $ 15,000 for the purchase of artificial limbs for soldiers and seamen disabled in the line of service, to be expended under the direction of the U.S. Surgeon General [8]. Four years later, in 1866, the War Department (now the DOD) was authorized to start a program which was to provide union veterans with transportation to and from their homes to a place where they could obtain their artificial limbs or devices and furnish those veterans with new artificial limbs or devices every five years [9]. The Veterans Bureau, a predecessor of the VA, was responsible for providing prostheses to World War I Veterans beginning in 1921 [9]. In May 2014, the FDA approved the DEKA prosthetic arm [10]. The significantly more advanced system developed by DEKA Integrated Solutions Corp. expands prosthesis choices for amputees, who have generally used body-powered prostheses—in particular, the split-hook device which was invented in 1912.
As for the legislative role of the VA, on 16 October 2017, the VA published a proposed rule in the Federal Register to revise the VA’s regulations governing the provision of prostheses, among other rehabilitative items and services to eligible veterans, known as Federal Regulation 84245 [11]. Coming into effect on 27 January 2021, the rule established that the VA is authorized to provide various categories of prosthetic and orthotic services, sensory aids, and medical devices to veterans nationwide as part of their active treatment and ongoing rehabilitation [11].
Federal Regulation 84245 reflects the VA’s more recent veteran-centered approach to health care, empowering veterans and clinicians to decide together which prosthetic equipment will best meet the veteran’s needs.
Previously, the provision of these services and devices varied across VA medical centers. According to VA Secretary Robert Wilkie [12], “It (the rule) ensures Veterans receive the same standard of service for the rehabilitative devices they need to live independently, no matter which medical center they walk into.” The rule reflects VA’s more recent veteran-centered approach to health care, by empowering veterans and clinicians to decide together which prosthetic equipment will best meet the veteran’s needs. While the equipment provided to veterans may not change much, the rule enables VA to identify current best practices to serve as the standard for all veterans who receive their care through the VA.
Defense Advanced Research Projects Agency (DARPA), a research and development agency of the DOD, is responsible for developing emerging technologies for military use. Benefitting from President Obama’s BRAIN Initiative, which started in 2013, DARPA launched the revolutionizing prosthetics program in 2014 [13]. The program aimed to gain FDA approval for an advanced electromechanical prosthetic upper limb with near-natural control, enhancing independence, and improving the quality of life for amputees [13].
Revolutionizing prosthetics is not DARPA’s only program pursuing the restoration of amputees. For example, the Agency’s hand proprioception and touch interfaces (HAPTIX) program seeks an alternative approach by using the peripheral nervous system to communicate motor commands and sensory feedback between the brain and a prosthetic limb [14] to restore the sense of touch. Originally created by Dr. Doug Weber, HAPTIX program is being operated under the auspices of DARPA’s Biological Technologies Office (BTO) [14]. It features a modular architecture and an extensible platform that provides a framework for future developments by themselves or others. The program was planned to initiate take-home trials of a complete, FDA-approved HAPTIX prosthesis system by 2019 [14].
While DARPA has done exciting work on arms and hands, the most remarkable breakthrough in foot, ankle, and leg prostheses has come out of medical academia. For instance, Hugh Herr, an American biophysicist at MIT, and his colleagues devised a new surgery: Agonist-antagonist myoneural interface (AMI) [15]. In the old amputation method, the connections between many agonist-antagonist groups are often severed, but AMI reconnects these pairs and builds new ones [15]. Artificial muscle electrodes are placed on each AMI pair and communicate with computers within the bionic limb. The contraction stretches the antagonist’s muscle, and these dynamics are communicated to the central nervous system, giving the person a sense of muscle length, speed, and force [16]. This sense is known as proprioception.
Proprioception is the body’s ability to sense movement, action, and location [17]. For example, when walking, this capability allows one to place their foot in front of the other without thinking about it. According to ClinicalTrials.gov, the AMI technique is still recruiting for its clinical trial phase and is not expected to be finished until February 2024 [18]. Therefore, it will be a while before the results are compiled and sent to the FDA for approval.
Dr. Herr was a champion mountain climber before losing both of his lower legs in a climbing accident. Nathan Copeland (Figure 1) regained some functions in his arms after 16 years of living with quadriplegia, as illustrated in Box 1 [19]. However, some people with certain disabilities may not directly benefit from these discoveries, such as those with cerebral palsy, and some may be more hesitant to consider cures like functional replacement of eyes and other organs. But what about the people who do want to improve or restore their quality of life?
In a 2016 study, a civilian volunteer named Nathan Copeland had been living with quadriplegia from the upper chest down since a 2004 car accident that caused a neck injury and spinal cord damage. After 12 years since the accident, Nathan willingly participated in clinical trials and underwent surgery to have four microelectrode arrays, each roughly half the size of a shirt button, implanted in his brain. Two of these arrays were placed in the motor cortex, while the other two were positioned in the sensory cortex region that corresponded with feeling in his fingers and palm. The prosthetic arm used in the study was equipped with advanced torque sensors capable of detecting pressure applied to any of its fingers and converted these physical sensations into electrical signals. These signals were then transmitted through the wires back to the arrays in Nathan’s brain, providing precise patterns of stimulation to his sensory neurons.
Nathan underwent surgery to have four microelectrode arrays, each roughly half the size of a shirt button, implanted in his brain.
In the initial set of tests, researchers gently touched each of the robotic fingers while Nathan was blindfolded. Remarkably, he could accurately report which finger was being touched with almost 100% accuracy. According to Nathan’s reports, the feeling he experienced was as if his own hand were being touched.
In June 2022, a young woman named Jordan Simpson (Figure 2) was working on her bachelor’s in social worker at the University of New England [20]. Ms. Simpson is a congenital amputee due to amniotic band syndrome. Jordan, also a passionate runner, was told by her insurance company back in Maine that it was not medically necessary to have a prosthesis better suited to running. Out-of-pocket costs for prostheses can range from $ 5,000 to $ 120,000. Fortunately, “An Act to Improve Outcomes for Persons with Limb Loss (L.D. 1003)” began to take shape after Maine State Representative Colleen Madigan (D-Waterville) visited one of Simpson’s social work classes [20]. The act requires that all health insurance policies regulated by the state of Maine must consider the recreational needs of children when deciding what prosthesis to cover.
Figure 2. Jordan Simpson running track in high school. Photo credit: University of New England.
This legislation is forward-thinking, especially because it is based on children’s quality of life, which appears to be an afterthought in many other fields. Children like to explore, and play, and learn who they are. Socializing among young peers can be more limited with a disability and this bill could grant normalcy to those of all ages without limbs and maybe other disabilities and defects.
In Maine, the “Act to Improve Outcomes for Persons with Limb Loss (L.D. 1003)” requires that all health insurance policies regulated by the state of Maine must consider the recreational needs of children when deciding what prosthesis to cover.
To conclude, other states need to follow Maine’s lead. With developments like HAPTIX and the AMI, the future looks bright for the people who will benefit from these advancements the most, and insurance companies nationwide need to adapt their policies to cover and support bionic technologies. We urge that there be a federal mandate to include bionics in federal insurance programs and insurance companies regulated by Obamacare. As prostheses advance in sensory capabilities, insurance companies also need to recognize prosthetic limbs as a part of the body susceptible to personal injury, not just as equipment, since they have haptic capabilities (i.e., the sense of touch) and can feel distress. This recognition would ensure better coverage and support for those with prosthetic limbs.
Author Information
Carolena Stephanie Larson is interested in food and water security issues as well as geopolitical and strategic factors in northeast and central Asia. As someone with a physical disability herself, she
is also very interested in human augmentation for military and civilian use. Larson has a master’s
in international security from the Schar School of Policy and Government, George Mason University, Arlington, VA 22201 USA. Email: clarso3@gmu.edu.
Muhammad Salar Khan is a Don E. Kash postdoctoral fellow in science and technology policy
at the Schar School of Policy and Government, George Mason University, Arlington, VA 22201 USA. His research centers around the intersection of policy, ethics, and innovation in artificial intelligence, with an emphasis on enhancing the technology’s explainability and comprehending its impact on economic and labor market outcomes, all with the ultimate aim of promoting the safe adoption of AI technology. Additionally, he explores the social implications of various emerging technologies. Furthermore, his research dives into local absorptive capabilities, especially technological knowledge sophistication, and their influence on economic growth in different countries. Khan has a master’s in public policy from Oregon State University, Corvallis, OR, USA, as a Fulbright Scholar and has a PhD in public policy from George Mason University.
______
To read the full version of this article, including references, click HERE.
_______







 JOIN SSIT
JOIN SSIT